The National Drug Code (NDC) is an identifier that may be associated with a Product; or, since the commercial package size is part of the NDC, each Inventory Item may have an NDC that is different.
The NDC is a free-form field, and is one of the 'Unique Inventory Attributes'.
Inventory Items may be located by their NDC using the following search screens:
‘Inventory Management’ screen
If using the NDC to location Inventory Items, the complete NDC must be used - partial search is not allowed.
The 10-digit NDC will be in one of the following configurations: 4-4-2, 5-3-2, or 5-4-1, meaning that there are 4 or 5 digits for the labeler code, 4 or 3 digits for the product code, and 2 or 1 digit(s) for the package code. The FDA maintains a searchable database of all NDC codes on their website.
More about National Drug Codes can be found on these websites:
National Drug Code Directory:
Electronic Animal Drug Product Listing Directory:
A free-form input field.
Contains the National Drug Code for a pharmaceutical Product in Inventory.
This field can be viewed or edited via these screens:
The Drug Listing Act of 1972 requires registered drug establishments to provide the Food and Drug Administration (FDA) with a current list of all drugs manufactured, prepared, propagated, compounded, or processed by it for commercial distribution. (See Section 510 of the Federal Food, Drug, and Cosmetic Act (Act) (21 U.S.C. § 360)). Drug products are identified and reported using a unique, three-segment number, called the National Drug Code (NDC), which serves as a universal product identifier for drugs. FDA publishes the listed NDC numbers and the information submitted as part of the listing information in the NDC Directory which is updated daily. The information submitted as part of the listing process, the NDC number, and the NDC Directory are used in the implementation and enforcement of the Act. (U.S. Food & Drug, 2019)
The NDC, or National Drug Code, is a unique 10-digit or 11-digit, 3-segment number, and a universal product identifier for human drugs in the United States.
The 3 segments of the NDC identify:
the labeler
the product
the commercial package size
What is a National Drug Code (NDC)?
The first set of numbers in the NDC identifies the labeler (manufacturer, repackager, or distributer). The second set of numbers is the product code, which identifies the specific strength, dosage form (i.e, capsule, tablet, liquid) and formulation of a drug for a specific company. Finally, the third set is the package code, which identifies package sizes and types. The labeler code is assigned by the U.S. Food and Drug Administration (FDA), while the product and package code are assigned by the company. For billing or other purposes, an NDC may also be arranged in an 11-digit format.
The NDC Directory is limited to all over-the-counter (OTC) medications, prescription medications, and insulin packages in the U.S. FDA publishes the listed 10-digit NDC numbers and the information submitted as part of the listing information in the NDC Directory which is updated daily. As of June 1, 2011, only drugs for which electronic listings (Structured Product Labeling or SPL) have been submitted to FDA are included in the NDC Directory. Animal drugs, blood products, or human drugs, among others, that are not in final marketed form are not included in the NDC directory.
How is the NDC formatted?
The 10-digit NDC will be in one of the following configurations: 4-4-2, 5-3-2, or 5-4-1, meaning that there are 4 or 5 digits for the labeler code, 4 or 3 digits for the product code and 2 or 1 digit(s) for the package code. The FDA maintains a searchable database of all NDC codes on their website.
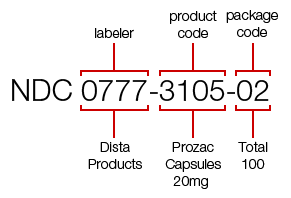
Example NDC
For example, the NDC for a 100-count bottle of Prozac 20 mg is 0777-3105-02. The first segment of numbers identifies the labeler. In this case, the labeler code "0777" is for Dista Products Company, the labeler of Prozac. The second segment, the product code, identifies the specific strength, dosage form (i.e, capsule, tablet, liquid) and formulation of a drug for a specific manufacturer. In this case, "3105" identifies that this dosage form is a capsule. The third segment is the package code, and it identifies package sizes and types. This example shows that the package code "02" for this bottle of Prozac identifies that 100 capsules are in the bottle.
Where can I find an NDC number for a drug?
The FDA maintains a searchable database of NDC codes on their website. NDC numbers can also be found in the drug product labeling (the package insert) as well as on the package itself.
Learn More: Use the Drugs.com Pill Identification Wizard to identify medications by NDC number.
Why are some drug products not in the NDC Directory?
According to the FDA, there are a number of reasons why a drug product may not appear in the NDC Directory, including:
The product may not be a prescription drug, OTC, or an insulin product.
The manufacturer has notified the FDA that the product is no longer commercially available and marketed.
The manufacturer has not provided a complete listing of the drug product.
As of June 1, 2011, only drugs for which electronic listings have been submitted to FDA are included in the NDC Directory. Drugs submitted via a paper form, prior to June 2009, are included on a separate file and will not be updated after June 2012.
Why do some NDC numbers have 11 digits?
For certain purposes, including the proper billing of drug products, an 11-digit NDC may be required. The Centers for Medicare & Medicaid Services (CMS) and other government entities require an NDC as part of their billing claim form. Some government agencies, including HIPAA, may require the NDC in an 11-digit format with leading zeros. Increasingly, private payers are requiring the 11-digit code, but rules can vary greatly.
NDC numbers have also appeared with an asterisk in either a product code or a package code. The asterisk acts as a placeholder and indicates the configuration of the NDC. Per the FDA, because of a conflict with the HIPAA standard of an 11-digit NDC, many programs will pad the product code or package code segments of the NDC with a leading zero instead of an asterisk. However, according to the FDA, asterisks are no longer used or included within the product file data elements to indicate certain configurations of the NDC.
Since a zero can be a valid digit in the NDC, this can lead to confusion when trying to return the 11-digit NDC back to its 10-digit FDA standard. For example, as noted by the FDA, 12345-0678-09 (11 digits) could be 12345-678-09 or 12345-0678-9 depending on the firm's configuration.
How do you convert a 10-digit NDC to an 11-digit NDC?
Increasingly payers are requiring an 11-digit NDC code for billing purposes. Therefore, proper billing may require a specially-placed zero to create a 5-4-2 format depending upon the drug product’s 10-digit NDC. See Table 1 for conversion examples. Note that hyphens for the 11-digit NDC (in the last column below) are for illustration purposes only, and should not be used when submitting data for a claim.
Table 1: 10-Digit to 11-Digit NDC Conversion
10-Digit Format on package10-Digit Format on packageConverted 11-Digit FormatActual 10-digit example11-digit conversion exampleTable 1: Adapted from Maryland Dept. of Health (www.maryland.gov)4-4-29999-9999-995-4-20777-3105-02 (Prozac)00777-3105-025-3-299999-999-995-4-243063-609-30 (alprazolam)43063-0609-305-4-199999-9999-95-4-211822-0544-1 (acetaminophen)11822-0544-01
How are NDC numbers used for billing purposes?
When submitting a claim for reimbursement, it is always best to check with the payer(s) to determine the specifics for NDA coding and reimbursement, as rules vary widely. According to the American Academy of Pediatrics (AAP), many payers like Blue Cross and Blue Shield, Tricare, and state Medicaid plans have guidance on how they want NDC codes to be used. In addition, some Medicaid plans exclude the use of NDC codes for vaccines.
Sources
National Drug Code (NDC) Directory. The U.S. Food and Drug Administration (FDA). Page Last Updated: 11/09/2017. Accessed February 5, 2018 at https://www.fda.gov/Drugs/InformationOnDrugs/ucm142438.htm
National Drug Code Database Background Information. The U.S. Food and Drug Administration (FDA). Page Last Updated: 03/20/2017. Accessed April 30, 2019 at https://www.fda.gov/drugs/development-approval-process-drugs/national-drug-code-database-background-information
Coding Corner: How to use National Drug Codes when billing for medications, vaccines. AAP News. The American Academy of Pediatrics (AAP) Division of Health Care Finance. 2016. Accessed February 5, 2018 at aappublications.org/news/aapnewsmag/2016/04/21/Coding042116.full.pdf
NDC Product File Definitions. The U.S. Food and Drug Administration (FDA). Page Last Updated: 12/08/2017. Accessed February 5, 2018 at https://www.fda.gov/Drugs/InformationOnDrugs/ucm254527.htm
National Drug Code Directory
Electronic Animal Drug Product Listing Directory